Resources for Public & Patient Involvement (PPI)
Public and Patient Involvement (PPI) is a way of doing research that supports collaboration between people with lived experiences, researchers, and research institutions.
Below, you’ll find some of the resources we’ve found most helpful on our PPI journey. We hope you find them useful, too! To add your own resource, or to suggest additions, please email the RDCTN PPI Liaison Officer, Dr Cassy Dinius at cassandra.dinius@ucd.ie.
Our work is shaped by the people it affects most. To learn more about PPI in RDCTN, click here to visit our ‘Public & Patient Involvement (PPI) Panel’ webpage.
To learn more about getting involved with the RDCTN PPI Panel, click here to visit our ‘Get Involved’ webpage.
Public and Patient Involvement (PPI) is a way of doing research that supports collaboration between people with lived experience and researchers. Here are a few of our favourite resources so you can learn more!
The Rare Disease Clinical Trial Network partners with contributors who have experience in any rare disease. They form our PPI Panel. Contributors are equal partners in research projects and improve the quality and relevance of research.
Wait, what was that term again? To keep things clear, we've put together a quick reference of common abbreviations. As always, if you’re unsure about anything, just ask!
This glossary was co-created with insights from the UCD Clinical Research Centre and individuals living with rare diseases. We extend our appreciation to the following whose contributions inspired this glossary: Health Research Charities Ireland, Health Research Board, and Prof. Rachel Crowley.
The resource was introduced at their first public engagement event in April 2023, “About us, By us…” ARUARES, The Apricot is an acronym which serves as a mental reminder/note when seeking to engage diverse communities. The members have shown that inclusivity does not always require extra resources but rather mindful consideration.
This guidance was developed by a group of public, patients, and researchers. We designed it to help you maximise the impact of your rare disease research poster.
Even small changes can make your research more approachable. Simple adjustments like improving the layout, using clear language, or adding visuals can make a big difference. We encourage you to try adapting one or two elements to help your work connect with as many people as possible!
In this unique interview, we ‘flip the script’ by having Public and Patient Involvement (PPI) contributor Liz Molloy lead the conversation with researcher Prof Rachel Crowley.
Every question in this interview was co-created by PPI contributors, ensuring that the topics reflect patient and public interests and insights. In this discussion, we explore the impact of PPI on research, the value of patient and public perspectives, and the importance of inclusivity in scientific work.
This video is part of our commitment to showcasing collaborative approaches in research and is available in multiple accessible formats (black and white, audio-only, transcript-only, and full color, all with captions).
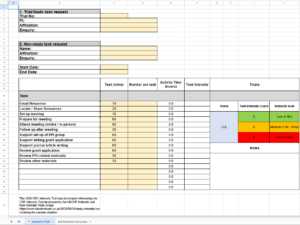
Wondering how time intensive your PPI Projects really are?
Network Partners